The periodic table is 150 years old this week
Companies, agencies, institutions, etc
Editor's
Lavoisiers’
Dalton
H2SO4
H2SO4 + Zn→ZnSO4 +
Volta
Newlands
the University of St Petersburg
Thomson’s
1913.Models
rhenium).Moseley
Quantum
Technetium
Seaborg
the University of California, Berkeley
Oganesson
The Economist
The Economist Newspaper Limited
People
Antoine-Laurent de Lavoisier
Marie-Anne
Louis-Joseph Proust
Antoine Lavoisier’s
John Dalton
Quaker
Jacob Berzelius
Alessandro Volta’s
Humphry Davy
Aluminium
Johann Dobereiner
Robert Bunsen
Gustav Kirchhoff
John Newlands
Albert Einstein
Dmitri Mendeleev
Rumplestiltskin”
Julian
William Ramsay
Henri Becquerel
J.J. Thomson
Ernest Rutherford
ones).Becquerel
Polonium
Pierre
Marie Curie
Actinium
Niels Bohr
Henry Moseley
Bohr-Rutherford
Glenn Seaborg
physicists’
Groups
Frenchman
Daltonism
Greek
Swede
German
Briton
Latin
Danish
American
Physical locations
Europe
Silicon
Places
No matching tags
Locations
France
Manchester
England
St Petersburg
Germany
Russia
Newlands
Alamogordo
New Mexico
Nagasaki
Japan
Events
No matching tags
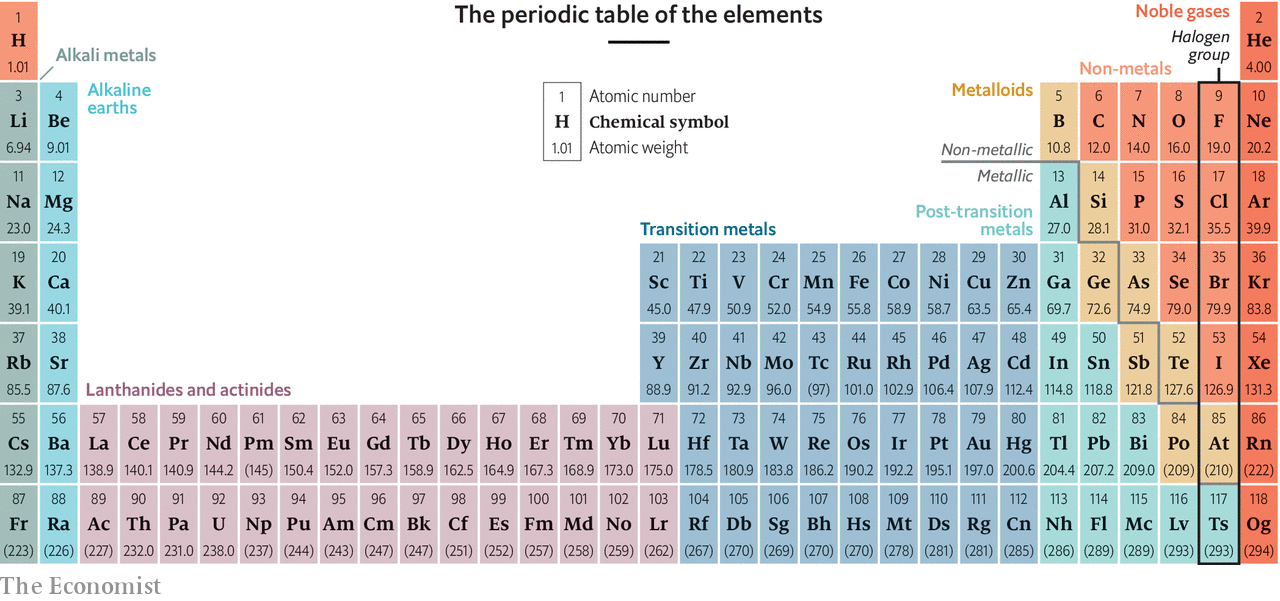
Summary
But Lavoisier’s laboratory is as good a place as any to begin, for it was Lavoisier who published the first putatively comprehensive list of chemical elements—substances incapable of being broken down by chemical reactions into other substances—and it was Lavoisier and his wife Marie-Anne who pioneered the technique of measuring quantitatively what went into and came out of a chemical reaction, as a way of getting to the heart of what such a reaction really is.Upgrade your inbox and get our Daily Dispatch and Editor's Picks.Lavoisier’s list of elements, published in 1789, five years before his execution, had 33 entries. Its rows and columns of rectangles, each containing a one- or two-letter abbreviation of the name of an element, together with its sequential atomic number, represent an order and underlying structure to the universe that would have astonished Lavoisier. This law, published in 1794, the year of Antoine Lavoisier’s execution, states that the ratio by weight of the elements in a chemical compound is always the same. In each case, if the members were arranged in order of atomic weight, the middle element (sodium, strontium, bromine, selenium) had a weight that was the average of the lightest and the heaviest of the three. Arranging the known elements in order of atomic weight, he believed he had discerned that, like a musical scale, every eighth element “rhymed” in the ways that sodium rhymed with potassium, and chlorine with bromine.The trouble with Newlands’ scheme was that an awful lot of the rhymes were forced. His brainwave was to recognise that, just as games of patience require the player to organise the pack as a grid of suits in order of the value of the cards, so the elements might be arranged by their atomic weights in “suits” that shared chemical and physical properties. In fact, it was a sign of strength—the more so because, for several of the gaps, he described in detail the properties of the elements he predicted would fill them, and these predictions were, by and large, fulfilled.Similarly, there are places in Mendeleev’s original table where it works only by cheating—that is, by swapping two adjacent elements between the places to which their atomic weights assign them. A few such pairs, cobalt and nickel for example (which actually share a slot in the published table), remained stubbornly out of kilter, providing evidence that atomic weight was really a proxy for some deeper structural principleCrucially, Mendeleev was not constrained, as Newlands had been, by preconceptions about how things ought to be. Helium, which Mendeleev ignored because its atomic weight could not be established, turned out to be the lightest member of a whole, new row (or column, in a modern table). Thus did Becquerel discover radioactivity.The following year, J.J. Thomson worked out that “cathode rays” emitted into a vacuum by a negative electrode were electrically charged particles that weighed far less than any atom. In the same year Henry Moseley, another of Rutherford’s confrères, found a mathematical relationship between an element’s X-ray spectrum when bombarded with electrons and its atomic number in the table. He also predicted two new transition metals, with atomic numbers 72 and 75, which duly turned up in 1923 (hafnium) and 1925 (rhenium).Moseley’s X-ray spectra demonstrated that an element’s atomic number does not depend directly on its atomic weight. Rutherford soon showed that the atomic number is actually the number in a nucleus of a positively charged particle that came to be known as a proton. Neutrons are also the reason that an element can have atoms of different atomic weights, known as isotopes. The colours of these lines represent energy released as light by electrons moving between orbitals.It is the shells that define the table’s rows. The fourth, from potassium to krypton, fills the s and p orbitals of the fourth shell and the d of the third shell (which has ten electrons altogether, for the ten columns of transition metals).Compounds are created either by unpaired electrons from different atoms forming joint orbitals called covalent bonds, or by the complete transfer of unpaired electrons between atoms, to create paired orbitals in the recipients. The repetitive order in which the shells are filled in each row means that elements in each column of the table have the same combination of unpaired electrons, and thus similar properties. Further analysis showed, moreover, that the difference between metals and non-metals depends on how easy an atom’s outer electrons are to detach (if easily detached, they can flow as an electric current, reflect light in the way that makes metals shiny, and confer ductility on the solid form of the element). But as Mendeleev himself said, “To conceive, understand and grasp the whole symmetry of the scientific edifice, including its unfinished portions, is equivalent to tasting that enjoyment only conveyed by the highest forms of beauty and truth.” For those who share this view, and see in the periodic table a supreme example of nature’s poetry, the row-completing, album-filling addition of oganesson may seem as good a place as any to stop.Join them.
As said here by